To begin, you will need a GDMS (Glow Discharge Mass Spectrometry) report. This can be used to make a preliminary estimate of the theoretical purity of silica (SiO₂). However, the following key limitations and calculation methods must be noted:
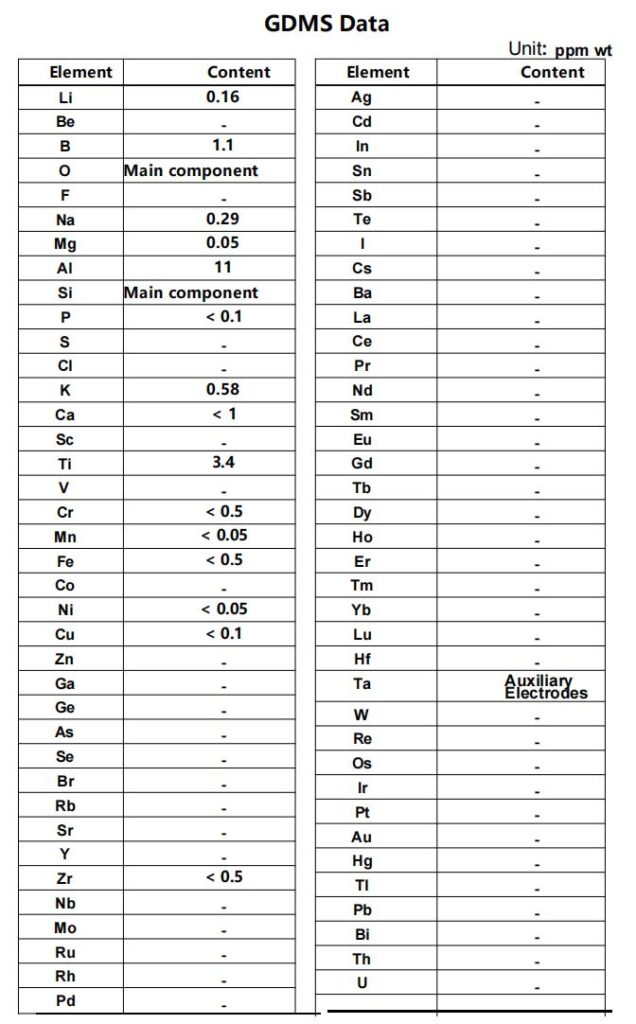
GDMS Report
1. Example Method for Estimating SiO₂ Purity
(1) Direct Calculation Method (Main Component Deduction Method)
Assumption:
The main component of the quartz tube is SiO₂, and all other elements are considered impurities.
Formula:
SiO₂ purity ≈ 100% − Σ (content of all impurity elements)
Calculation Steps:
- Extract from the report the contents of all impurity elements other than oxygen and silicon (in ppm by weight).
- Add up the impurity contents to obtain the total impurity content.
- Subtract the total impurity percentage (ppm ÷ 10,000) from 100%.
(2) Actual Calculation (Based on Report Data)
Determine the total amount of detected impurities (unit: ppm):
Li (0.16) + B (1.1) + Na (0.29) + Mg (0.05) + Al (11) + Ti (3.4) + K (0.58) + other upper limits (e.g., Fe <0.5, Cr <0.5…)
≈ 0.16 + 1.1 + 0.29 + 0.05 + 11 + 3.4 + 0.58 + 0.5 (Fe) + 0.5 (Cr) + 0.05 (Ni) + 0.1 (Cu) + 1 (Ca)
≈ 18.73 ppm (conservative estimate, calculated using upper limits)
SiO₂ Purity:
100% − (18.73 ÷ 10,000) = 99.8127%
(3) Correction Factors
- Undetected elements: Elements marked as “–” in the report (such as Au, Hg, etc.) may be below the detection limit of the instrument, but are not counted in the total impurities.
- Oxygen content not quantified: The report only marks oxygen as a “main component,” but in actual SiO₂, oxygen accounts for 53.2% of its composition (stoichiometric ratio correction is required).
2. Purity Evaluation Conclusion
- Conservative purity: ≥ 99.81% (calculated using the upper limits of GDMS-detected impurities)
- Actual purity may be higher: If some elements are well below their upper limits (e.g., Fe is only 0.1 ppm), the purity could reach 99.9%.
3. Key Limitations and Considerations
(1) Limitations of the GDMS Method
- Semi-quantitative data: For example, Fe <0.5 ppm might actually be 0.1 ppm or 0.01 ppm, which can significantly affect purity calculation.
- Missing light elements: GDMS has weak detection capability for light elements like C and H, which may lead to underestimating total impurities (e.g., hydroxyl OH⁻ not detected).
(2) Comparison with Industry Standards
| Material Grade | Typical SiO₂ Purity Requirement | Purity Estimated from GDMS | Meets Standard |
|---|---|---|---|
| Industrial-grade quartz | ≥99.5% | 99.81% | ✅ Yes |
| Photovoltaic-grade quartz | ≥99.9% | Close, but uncertain | ⚠ ICP-MS verification needed |
| Semiconductor-grade quartz | ≥99.99% | Not achieved | ❌ No |
4. Application Recommendations for the Material
- Industrial/general use: Purity of 99.8% is sufficient and can be used directly.
- Photovoltaic/semiconductor use:
- Use ICP-MS to confirm whether critical metallic impurities (Fe, Na, etc.) are truly below 0.1 ppm.
- Supplement with FTIR testing to determine hydroxyl content (OH⁻ < 5 ppm).
Summary:
This material belongs to high-purity industrial-grade quartz but does not meet semiconductor-grade requirements (4N5 or 5N).
Decision basis:
If the customer’s process is sensitive to Fe/Na contamination (e.g., PERC solar cells), priority should be given to verifying the actual impurity levels.


