This is a very practical and important question. Quartz tubes serve as the core reaction chamber of a CVD tube furnace, but not every experiment strictly requires them. Their necessity depends mainly on the temperature, atmosphere, and chemical compatibility involved.
Below are the experimental scenarios where quartz tubes are mandatory or highly recommended, along with the scientific reasons behind these requirements.
Experiments Where Quartz Tubes Are Mandatory or Strongly Recommended
1. High-Temperature Oxidation Experiments (>1000°C)
Typical experiments:
Growth of SiO₂ films through silicon thermal oxidation; high-temperature annealing in oxygen or air atmospheres.
Reason:
The main component of quartz tubes is silicon dioxide (SiO₂), which is extremely stable under high-temperature oxidizing environments. It does not react, shed particles, or contaminate samples.
In contrast, metal tubes would oxidize instantly and become damaged.
2. Experiments Involving Hydrogen-Containing Reducing Atmospheres
Typical experiments:
Reduction of metal oxides (e.g., reducing CuO to Cu), graphene growth (CH₄/H₂ gas mixture), annealing of certain metals.
Reason:
High-purity quartz has very low hydrogen permeability and exhibits excellent resistance to hydrogen at high temperatures.
Although prolonged exposure to hydrogen at high temperatures may cause slight “devitrification” (becoming opaque and brittle), quartz still performs far better than most metals, which react with hydrogen or undergo hydrogen embrittlement.
3. Growth of Materials That Are Extremely Sensitive to Metal Contamination
Typical experiments:
Semiconductor material growth (Si, Ge epitaxy), high-purity graphene, hexagonal BN (hBN), 2D TMDs such as MoS₂ and WS₂.
Reason:
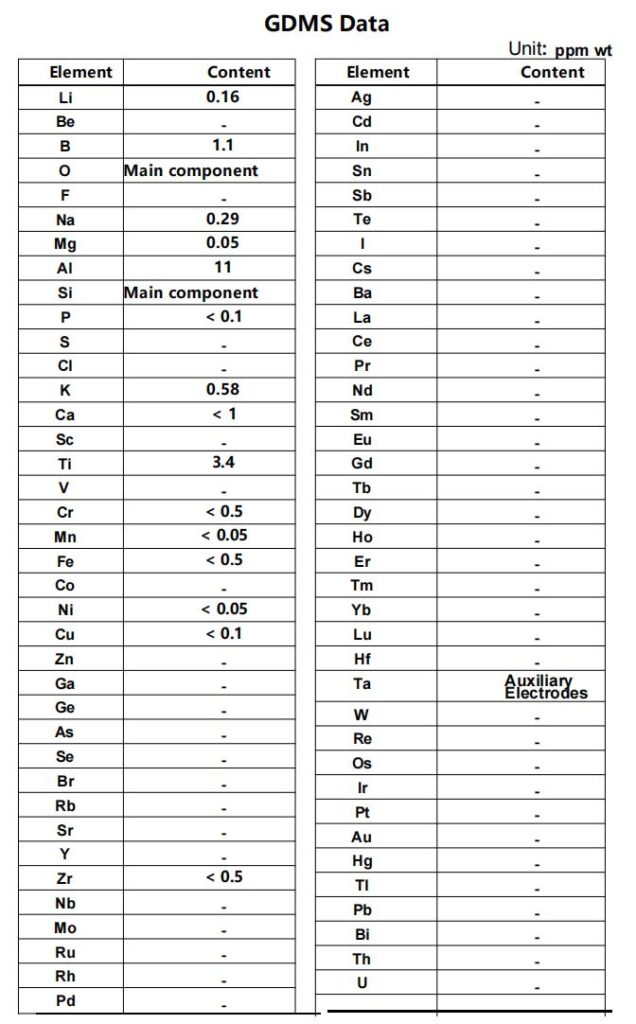
High-purity quartz contains extremely low levels of metallic impurities (ppm level).
Metal furnace tubes (such as stainless steel or Inconel) release trace metal atoms at high temperature. These atoms can deposit on samples and become defect centers, severely degrading semiconductor electrical properties (e.g., carrier mobility) and optical performance.
4. Experiments Requiring Visual Observation
Typical experiments:
Observing intermediate reactions during CVD processes, monitoring sample color changes with temperature, educational demonstrations.
Reason:
Quartz glass is transparent, allowing direct visual monitoring of the reaction inside the tube. This is highly valuable in process development and troubleshooting.
5. Experiments Using Strongly Corrosive Precursors
Typical experiments:
CVD processes using halides such as WF₆ or TiCl₄ as precursors.
Reason:
Quartz has excellent resistance to acidic corrosion. Although it is not resistant to HF or hot phosphoric acid (H₃PO₄), its tolerance to most other halides and acidic gases is much higher than that of metal materials.
Experiments That Do Not Require or Are Not Suitable for Quartz Tubes
1. Ultra-High-Temperature Experiments (>1250–1300°C)
Reason:
Although quartz has a high softening point, extended operation above 1200°C accelerates devitrification and deformation, increasing the risk of tube failure.
In such cases, alumina or silicon carbide (SiC) ceramic tubes should be used.
2. Experiments Involving Alkali Metals
Reason:
Quartz (SiO₂) reacts with alkali metals (Na, K) and their vapors at high temperatures, forming silicates. This severely corrodes and damages quartz tubes.
Such experiments require stainless steel tubes lined with a protective coating.
3. Experiments Using Fluorine-Based Chemicals
Reason:
Quartz reacts with HF or F₂ (SiO₂ + 4HF → SiF₄↑ + 2H₂O), leading to tube corrosion.
Sapphire tubes or coated stainless steel tubes must be used.
4. Ultra-High Vacuum (UHV) Systems or Setups Requiring Magnetron Sputtering Attachments
Reason:
These systems typically use metal chambers to support welded flanges and ports for better sealing and mechanical connection.
Quartz tubes rely on O-ring sealing, and their achievable vacuum level is generally inferior to full-metal systems.
Core Conclusion
When your experiment requires high temperatures, oxidizing/reducing atmospheres, and extremely high purity, quartz tubes are virtually the only choice. Their high purity, chemical inertness, and optical transparency are irreplaceable advantages.
When selecting furnace tubes, always evaluate the chemical compatibility with your process gases and operating temperature.